Clinical pharmacovigilance
PV no longer a hurdle for your smooth clinical study
Running a clinical trial study
is often pain in and of itself.
Every bit of administrative overhead and complexity make the task and budget ever more daunting.
We make sure pharmacovigilance requirements won’t stand in the way of successfully managing your clinical studies.
Processing SAEs or SUSARs
does not need to be a soul-drenching activity
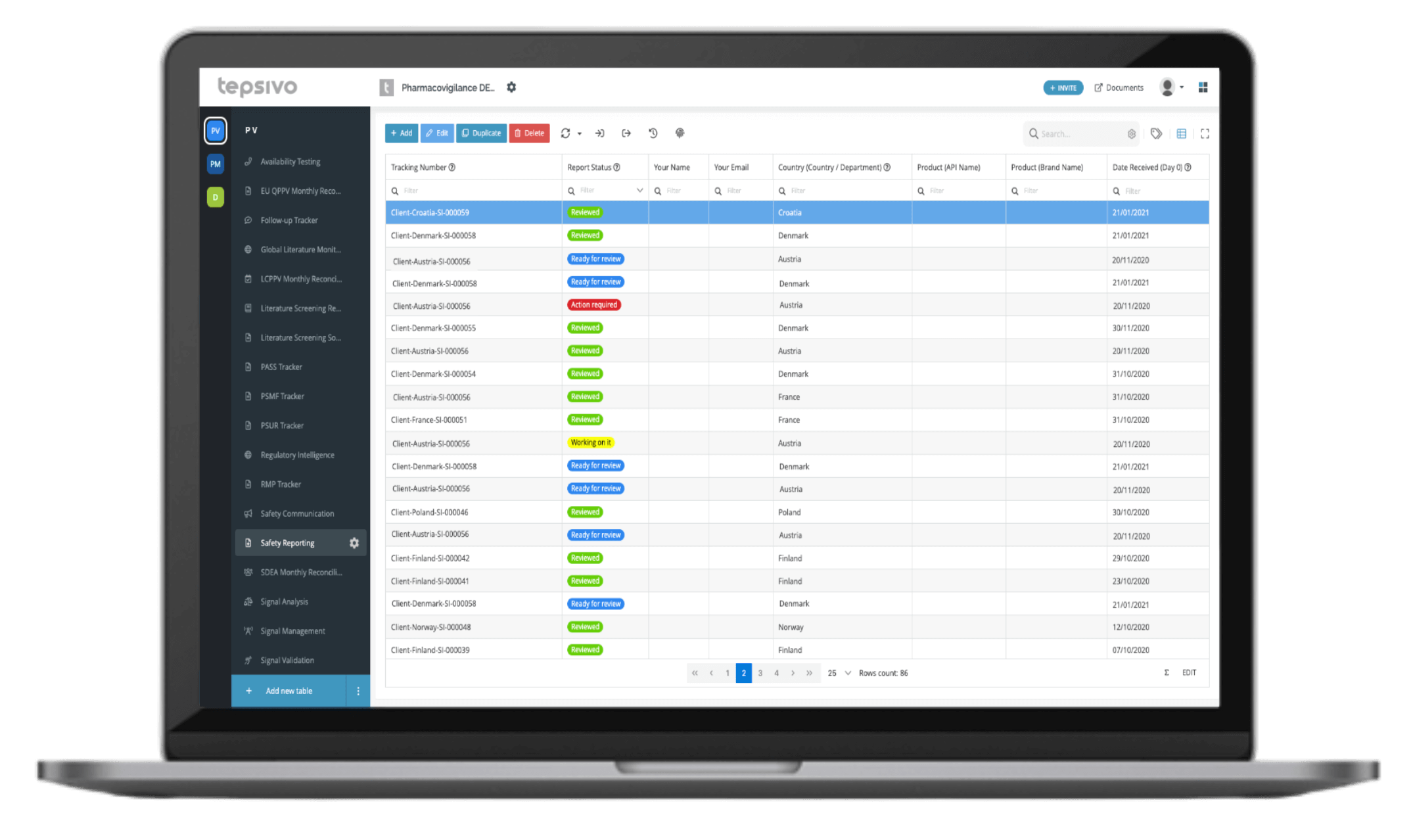
With its built-in case intake module, aggregate reporting and Tepsivo Safety Database, Tepsivo OnePV can take care of the full process with little human touch from A to Z.
100%
PV processes integrated in one system
0
administrative overhead or needless “PM meetings”
2
weeks to implement the full solution
Data under full control
and in the right format
- investigators report directly into Tepsivo OnePV
- file-to-text automatic transcriptions
- easy and direct reporting to FDA, EMA, MHRA and other authorities around the globe
- automated DSURs or other aggregate reports
End-to-end
Outsourced clinical process
Global
150+ countries covered
Software & Service
Combined in one unique solution
Focus on science
and value-added activities
By automating away and eliminating needless mundane tasks, we have no need to engage people for low-level administrative tasks and looking for lowest costs to be economical.
As a result, our pharmacovigilance team consists of highly qualified experts who focus their time where it should be spent – solely on activities with real impact on patient safety.
Tepsivo
OnePV
Clinical PV service is driven by Tepsivo OnePV and the fully integrated pharmacovigilance system, world’s only completely digital inhouse solution.
Tepsivo
OnePV
Clinical PV service is driven by Tepsivo OnePV and the fully integrated pharmacovigilance system, world’s only completely digital inhouse solution.
A single solution
for your PV needs
With Tepsivo OnePV utilized completely for a post-marketing pharmacovigilance system, your transition into approval marketing stages can be seamless through one system.
You can also run clinical studies in parallel with your existing marketing PV requirements, all managed completely by Tepsivo and Tepsivo OnePV.
1 provider
with inhouse software and full service
100% compliance
at the lowest possible cost
1 provider
with inhouse software and full service
100% compliance
at the lowest possible cost
Let’s have a chat
Whatever your needs are, we look forward to getting in touch with you.
Feel free to drop us a message and we will contact you right away.
Tepsivo Oy | Haartmaninkatu 4, Building 14, 00290, Helsinki, Finland | VAT number FI31367614 | contact@tepsivo.com | +358 402 204 698 | Privacy policy