Pharmacovigilance Literature Monitoring
Tepsivo Literature is the world’s largest automated medical literature screening
Literature monitoring in PV had always been
a time-consuming activity, especially on the local level
Back in 2022, we decided to change the status quo. We released Tepsivo Literature, the first user-friendly product that could screen through both global and local sources automatically.
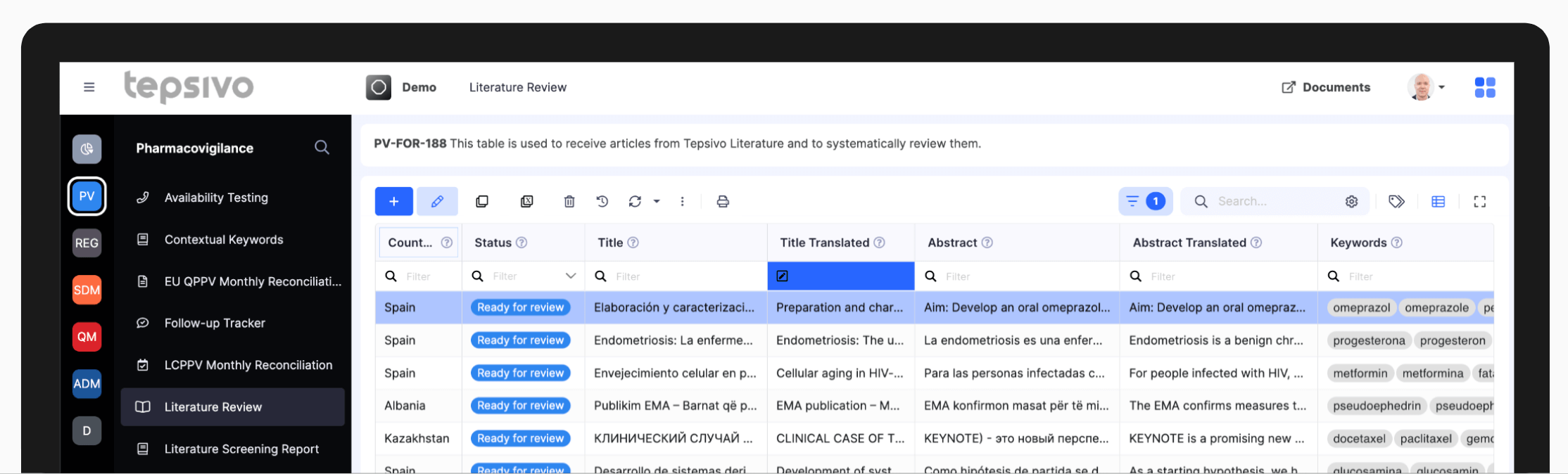
Today, the solution is still unmatched
It improves further with new sources regularly added, new products ready to switch on within minutes, and new features such as AI assessment to reduce further the human effort.
The largest dataset of sources for PV monitoring
Integrated AI replaces human work
Tens of thousands of articles screened efficiently
Going through tens of thousands of articles
has never been easier

- Tepsivo Literature now runs searches through tens of thousands of individual sources in local languages across 150+ countries, as well as large “global” databases
- We constantly review that it is the largest dataset you can find, ensuring 100% legal compliance in any market
And with complete automation, assisted by AI, we do this at the lowest possible cost.

The 1st among all literature monitoring solutions
in size, quality and cost
100 EUR / month per country
all-inclusive cost
100%
legal
compliance
150+
markets with
service available
10s of thousands
individual
sources covered
0
admin
overhead
100%
legal
compliance
150+
markets with
service available
10s of thousands
individual
sources covered
0
admin
overhead
And your product is already monitored
Yes, we mean it. If it is on the market, we already have it in our database. Just plug in! Ready to start within minutes.
Powerful integration with Tepsivo OnePV
Tepsivo Safety Database is E2B R3, authority-gateway connected solution, fully immersed within the Tepsivo OnePV environment.
As such, you can expect major benefits from a seamless integration with the original case intake, reconciliation, or retrospective signal analysis and aggregate reporting.
service
150 + countries
Reporting
Intelligence
OnePV
Management
Powerful integration with Tepsivo OnePV
Tepsivo Literature alone is more sophisticated than any other literature monitoring solution.
And that is before we even get to the benefits of using its complete integration within Tepsivo OnePV.
Virtually touchless AE reporting
straight to authority in a few, automated steps
1) Tepsivo Literature automation picks up any relevant hits by keywords
2) Auto-translates
3) AI assesses relevancy of the hit
4) Creates a safety report in the case intake part of Tepsivo OnePV
5) If an ICSR, automatically populates E2B R3 Tepsivo Safety Database
6) Sends to authority
with human expert oversight for QC and customer comfort
Tepsivo Literature automation picks up any relevant hits by keywords
AI assesses relevancy of the hit
If an ICSR, automatically populates E2B R3 Tepsivo Safety Database
Auto-translates
Creates a safety report in the case intake part of Tepsivo OnePV
Sends to authority
with human expert oversight for QC and customer comfort
And a lot more benefits
for your smooth PV operations
- All reports available for retrospective analysis within Tepsivo OnePV at any time
- Non-ICSR information directly linked to aggregate reporting or signal analyses
- Outputs always visible to EU QPPV, local QPPV’s, or local affiliates by country
Ad hoc searches
available for outputs
Complete audit trail
for inspection readiness
Ad hoc searches
available for outputs
Complete audit trail
for inspection readiness
Let’s have a chat
Whatever your needs are, we look forward to getting in touch with you.
Feel free to drop us a message and we will contact you right away.
Tepsivo Oy | Haartmaninkatu 4, Building 14, 00290, Helsinki, Finland | VAT number FI31367614 | contact@tepsivo.com | +358 402 204 698 | Privacy policy