No time to read now?
-> Download the article as a handy pdf
List of contents
What is DADI?
What is the Digital Application Dataset Integration Project?
Martti Ahtola & Sabiha Rahman Mim | Mar 24, 2022

Goodbye to pdf forms
The goal of the European Medicines Regulatory Network’s DADI project is to replace the current PDF-based electronic application forms with web forms[i]. DADI is part of the Heads of Medicines Agencies (HMA) eSubmission Roadmap[ii] and EU Telematics portfolio. The digitalization of the forms will apply to the majority of the forms currently created with the .pdf templates:- Variations
- Initial marketing authorization applications
- Renewals (human)
- Other submissions are under consideration, human and veterinary forms centrally authorized product (CAPs) and nationally authorized product (NAPs) applications
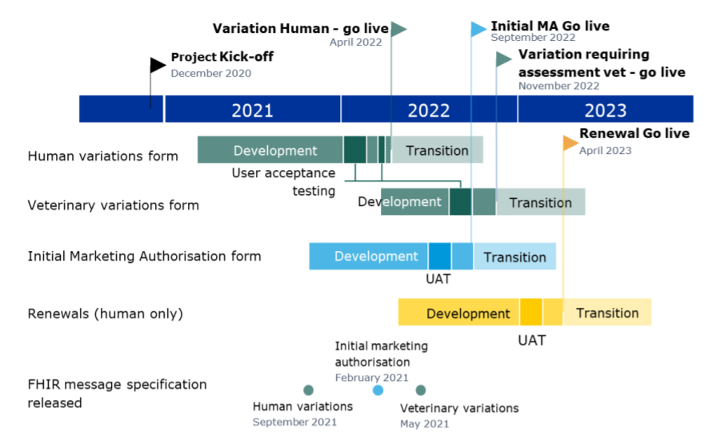
DADI Project Timeline (version August 2021)
Aim of DADI Project
The aim of the DADI project is to make electronic application form (eAF) completion and submission to authorities more efficient[iv].
To achieve this goal, the following steps will need to be achieved:
- Replace current PDF-file based input eAFs with web forms on an updated portal.
- Completing the forms produces both the familiar human-readable (.pdf) output and a new machine-readable output for digital processing (FHIR data exchange standard for medicinal products – more about FHIR below).
- Structure input of eAFs to capture standard product data (Product Management Services, PMS), ensuring ISO IDMP compliance. Reuse PMS data to prepopulate application inputs wherever relevant.
- Integration of the forms with improved processes for handling CAPs submissions, reducing manual processing, errors, and processing time.
The advantages EMA is hoping to get out of this digital transformation are quite generic and high-level (better decision-making using AI, transformation of processes etc.)
But of course, any digitalization EMA is aiming to achieve, is very welcome.
Background to the DADI Project
The work on replacing the eAFs with forms that would support efficiency and better interoperability started initially with the Common European Single Submission Portal (CESSP) Phase 1 project in 2016. CESSP has been apparently since rebranded and when the project ended in 2020, most of the features were postponed[v]. As stated by the authorities, the need to replace the forms has only increased since then as the current .pdf forms are aging, and “risk no longer being fit for purpose”. This is not the only technology that the Agency is responsible for that is soon becoming obsolete and it seems that EMA has currently a very wide range of systems updates coming up in the near future. EMA decided to build on the “momentum, relevant expertise and know-how” that was accumulated during the CESSP Phase 1 project and started another project: DADI. Essentially, the DADI project is the “CESSP Phase 2”. The original plan was that eAF variations and renewals would be submitted using the single submissions portal by the end of 2021, using the “CESP Application Dataset Management Module (CESP Dataset Module)”[vi].
The plans for eAF in June 2019 (vii)
System Description
The project brings a solution for electronic application form that works for the applicants (or marketing authorization holders, MAH) on the front end and for the EMA and NCAs on the back end. In the DADI project, EMA and the NCAs together are the two “product owners” for the development of the new forms. Together, they have formed a requirements group that:- Consists of subject matter experts representing EMA, NCAs and the pharmaceutical industry
- Provides expert insight into the use of forms
- Supports testing of the forms
What will change technically in the forms?
The main thing is, of course, that the applicants fill out a web form instead of the interactive PDF form. Anyone who has used the current .pdf version of eAF knows that at best the form filling is slow. At worst, you might be facing a long list of problems in addition to the slow repetitive work.
The output is currently a filled in .pdf form which is included in the electronic Common Technical Document (eCTD) / VNeeS package which is submitted to the regulators. After the implementation of the electronic forms, the output will include both the same human readable .pdf form and, in addition, a machine readable HL7 FHIR message which can be read and processed by IT systems[viii].
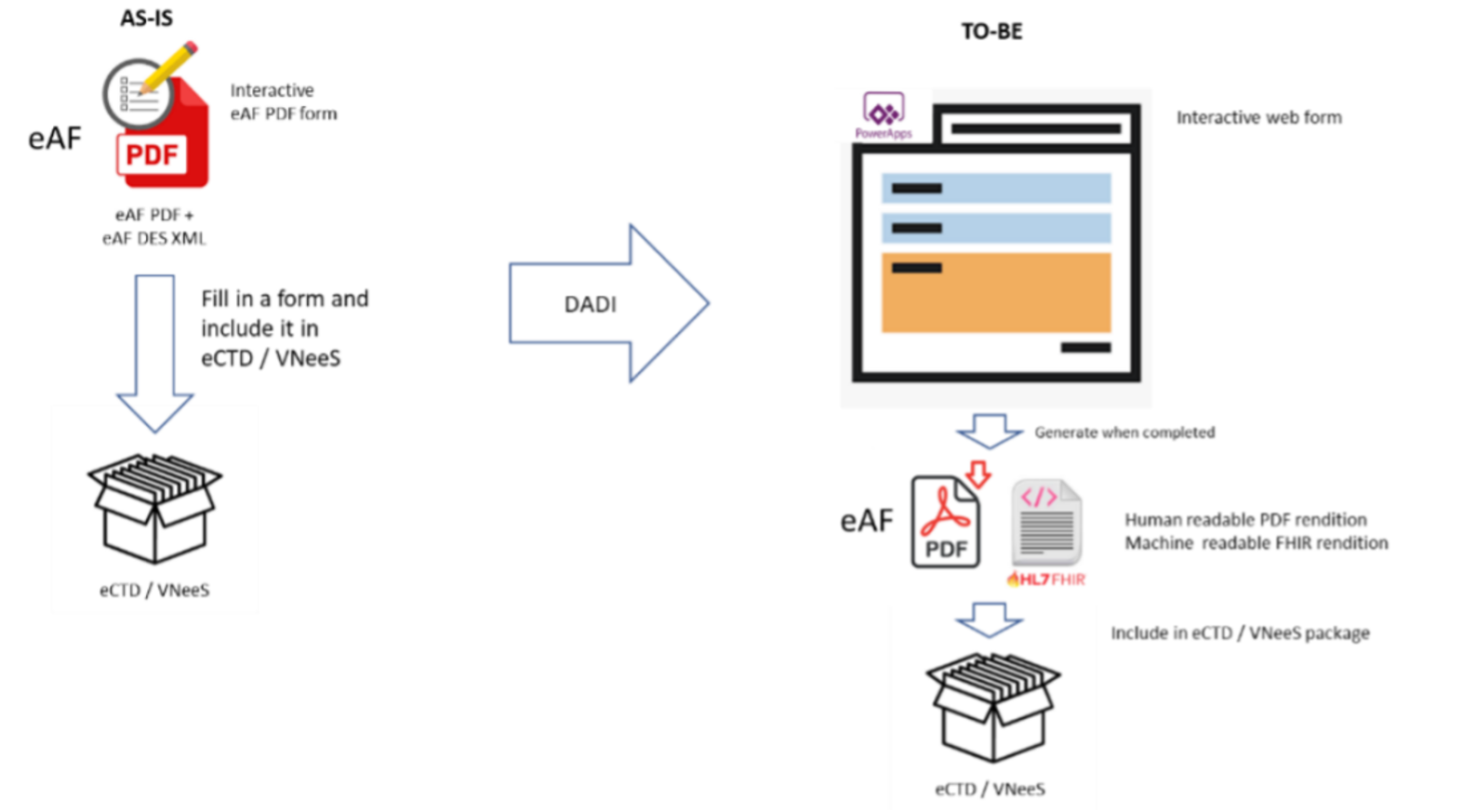
Comparison of current eAF process to the process after DADI
So, the main thing is the update of the input and the new FHIR document that can be further used in other systems of the authorities. For the pharmaceutical companies, the update should bring at least slightly faster and easier submission of variations, applications, and renewals (especially if the pre-population of fields and connection to SPOR elements work as planned). To the authorities this upgrade should give possibilities to automate and integrate and, to achieve the hoped for standardization of data.
How will the project impact industry stakeholders?
There are a set of features that are going to be implemented at one point or the other during the DADI project. These include for example the collection and use of standardized PMS/UPD (Union Product Database) product master data, pre-populating fields with available PMS/UPD data and industry having full visibility of data available on the regulator’s side.
The variations form will soon be in use, and the list of features have been made available[ix]. The list includes for example:
- Organization Search (connected to OMS)
- Product Selection (connected to IRIS and PMS)
- Including proof of payment
- Contextual guidelines for sections and fields and form validation
- Possibility to copy / duplicate application
- Tracked changes
Once the variation web form moves to testing and later, release and implementation a more detailed breakdown of possible impacts for industry will be made available.
In later releases, the aim is to eventually integrate eAF and data submission on authorized medicines (XEVMPD / Article 57 data) submissions into a single process. This would reduce unnecessary administrative burden and would improve the product data quality overall.
How will the DADI project impact NCAs?
The online application forms will be common for both NAPs as well as CAPs which are processed by NCAs and EMA respectively. The forms impacted are used in NCA relevant authorization procedures:- Mutual Recognition Procedure (MRP)
- Decentralized Procedure (DCP)
- National Procedure (NP)
- Subsequent Recognition Procedure (SRP) for veterinary
What is the connection between SPOR and DADI?
PMS and OMS are utilized in the new eAF online forms to improve the data quality and to link the variations and applications to the organizations across the different platforms used by the authorities. They are two of the four data management services managed by substances (SMS), products (PMS), organizations (OMS), and referentials (RMS) (SPOR) data management services for human and veterinary medicinal products. The goals of SPOR services include for example:- Increased data quality and simplification of data management practices, since data will be reviewed, assessed and approved as part of the new data operating model
- More efficient regulatory action and decision-making, thanks to improved data integrity and reliability
- Regulatory requirements can be met more effectively, by reducing data silos and improving interoperability across EU systems
- Operational savings and efficiencies can be achieved, as pharmaceutical companies need to supply regulatory data only once, which will be reused across different procedures and regulators.
What is FHIR?
Fast Healthcare Interoperability Resources (FHIR) was selected as the application programming interface for the PMS service, and the web-based eAFs are an example of FHIR being used to exchange product master data[x]. FHIR is the data exchange standard chosen to support the easy exchange of data between the different forms, systems and product databases such as eAF and PMS. Health Level Seven’s (HL7) FHIR is a standard for exchanging healthcare information electronically[xi]. FHIR is used in the new online forms in the following way:- The DADI project team creates a FHIR specification as the backbone of the new online form.
- FHIR aims to simplify implementation without sacrificing information integrity. It leverages existing logical and theoretical models to provide a consistent, easy to implement, and rigorous mechanism for exchanging data between healthcare applications.
- The basic building block in FHIR is a “Resource”. All exchangeable content is defined as a resource that contains a common way to define and represent them, a common set of metadata and a human readable part.
What’s the next step?
EMA and NCAs are teaming up to provide support, guidance, and training for applicants. Details should be shared closer to the roll out of the first form (Variations), expected early 2022. As part of the roll out of the new forms training will be made available and the exact format of the variation form should be determined. Several webinars are planned to support the rollout and to answer the questions from the industry. The latest update on the project is from 10th of August 2021[xii], which promises the next update in September 2021. Adding this to the previous delays, it can be expected that the rollout might not happen during early 2022. However, we remain hopeful, as the update of the variation and application processes would finally bring them to the 21st century. Tepsivo will follow the situation and when updated information is available, we will comment and act on it. In case you have questions about implementing the new online forms to your processes or regulatory submissions in general, contact us for some honest regulatory advice.Resources
[i] Summary of the Digital Application Dataset Integration Project (DADI) for partners and stakeholders http://esubmission.ema.europa.eu/cessp/DADI%20Project%20summary%20presentation.pdf
[ii] eSubmission expert group documentation http://esubmission.ema.europa.eu/tiges/cmbdocumentation.html
[iii] Digital Application Dataset Integration (DADI) http://esubmission.ema.europa.eu/cessp/cessp.htm
[iv] Digital Application Dataset Integration (DADI) Project Question and Answers http://esubmission.ema.europa.eu/cessp/DADI%20Questions%20and%20Answers.pdf
[v] eSubmission expert group documentation http://esubmission.ema.europa.eu/tiges/cmbdocumentation.html
[vi] Replacement of the current PDF electronic application forms (eAFs) with the CESP Application Dataset Management Module (CESP Dataset Module) http://esubmission.ema.europa.eu/tiges/docs/Annex%204%20to%20eSubmission%20Roadmap%20on%20eAF%20and%20CESSP.pdf
[vii] Updated eSubmission Roadmap (v2.2) http://esubmission.ema.europa.eu/tiges/docs/eSubmission%20Roadmap%20final%20adopted%20HMA.pptx
[viii] Digital Application Dataset Integration (DADI) Project Question and Answers http://esubmission.ema.europa.eu/cessp/DADI%20Questions%20and%20Answers.pdf
[ix] DADI eAF Project List of Features for the Variations web-based form http://esubmission.ema.europa.eu/cessp/DADI%20Feature%20List.pdf
[x] Digital Application Dataset Integration (DADI) http://esubmission.ema.europa.eu/cessp/cessp.htm
[xi] Introducing HL7 FHIR https://hl7.org/FHIR/summary.html
[xii] Digital Application Dataset Integration (DADI) http://esubmission.ema.europa.eu/cessp/cessp.htm
Did you like the article? Share with your network!
…or tell us your opinion.
Follow our newsletter!
Keep up with industry trends and get interesting reads like this one 1x per month into your inbox.Learn more about Tepsivo
We deliver modern PV solutions to fulfill your regulatory needs using less resources. See how we do it >

0 Comments